
ViroMed
ViroMed Co., Ltd. (ViroMed), a leading biopharmaceutical company focusing on the development of innovative medicine, was established in 1996 and is headquartered in Seoul, Korea with a US presence in Atlanta Area. ViroMed has been listed on the KOSDAQ stock exchange (084990) since 2005. ViroMed has assembled a diverse pipeline of novel biologics and Botanical therapeutics in the areas of cardiovascular disease, cancer and immune disorder, with eight projects in clinical stages in the US, Korea, and China. The company has established several key partnerships with Reyon Pharm. (Korea), PMG Pharm. (Korea) and Beijing Northland Biotech (China). The founder of ViroMed is Professor Sunyoung Kim of Seoul National University (SNU), who holds academic degrees from SNU, MIT, Harvard and Oxford, and also served as an assistant professor at Harvard Medical School and a postdoctoral fellow at MIT. Rallied under the leadership of Prof. Kim, experts from various fields have been united to achieve one goal – to cure the incurables.
Company Introduction

ViroMed Co., Ltd. (ViroMed), a leading biopharmaceutical company focusing on the development of innovative medicine, was established in 1996 and is headquartered in Seoul, Korea with a US presence in Atlanta Area. ViroMed has been listed on the KOSDAQ stock exchange (084990) since 2005.
ViroMed has assembled a diverse pipeline of novel biologics and Botanical therapeutics in the areas of cardiovascular disease, cancer and immune disorder, with eight projects in clinical stages in the US, Korea, and China. The company has established several key partnerships with Reyon Pharm. (Korea), PMG Pharm. (Korea) and Beijing Northland Biotech (China).
The founder of ViroMed is Professor Sunyoung Kim of Seoul National University (SNU), who holds academic degrees from SNU, MIT, Harvard and Oxford, and also served as an assistant professor at Harvard Medical School and a postdoctoral fellow at MIT. Rallied under the leadership of Prof. Kim, experts from various fields have been united to achieve one goal – to cure the incurables.
Detailed Company Information
ViroMed
- Business Type Manufacturer
- Year Established 1996
- Location South Korea
- Main Markets
- Total Employees 51-100 People
- Homepage http://viromed.co.kr/main/
- President Yongsoo Kim
- Phone +82-2-2102-7298
- FAX +82-2-873-8022
- Address #5, Bldg, 203, Seoul National University, 1, Gwanak-ro, Gwanak-gu, Seoul 151-742, Korea
-
Product Category
Beauty & Personal Care > Personal Care > Facial Care > Skin Care Set
Health & Medical > Health > Health Products > Health Care Supplement - Factory Information
Additional Introduction
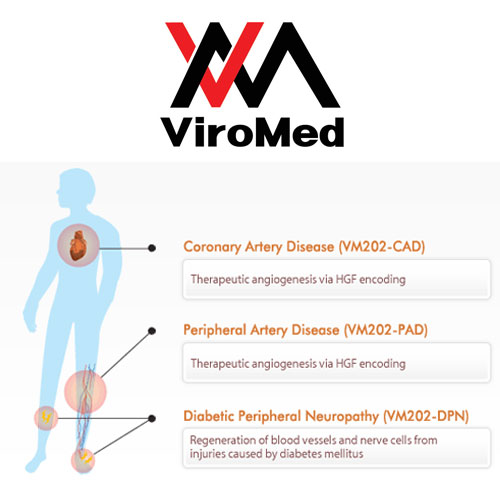
ViroMed is headquartered in Seoul, Korea with a US presence in the Atlanta area. The company has assembled a diverse pipeline of novel biologic and herbal therapeutics in the areas of cardiovascular disease, cancer, and immune disorder, with a total of twelve clinical trials ongoing in the US, Korea, and China. ViroMed is publicly held with a listing on the KOSDAQ stock exchange (084990).
Our History
- FEB, 2014 Orphan drug designation by FDA for Amyotrophic Lateral Sclerosis (VM202-ALS)
- FEB, 2014 Domestic launch functional food based on HX106 & red ginseng, a nutraceutical designed to improve working memory
- DEC, 2013 Phase I clinical study of VM206RY completed in Korea
- NOV, 2013 Collaborative development agreement for Amyotrophic Lateral Sclerosis (VM202-ALS) with Reyon Pharmaceutical Co.,Ltd. in Korea
- OCT, 2013 Phase I/II clinical study of VM202-ALS (Amyotrophic Lateral Sclerosis medicine) approved by US FDA.
- DEC, 2012 Murphin (functional food) launched
- DEC, 2012 Osteoarthritis medicine PG201 (Layla) launched in Korea (marketed by PMG Pharmaceutical)
- JUL, 2012 Chronic Hepatitis B (HBV) vaccine licensed out to Reyon Pharmaceutical
- MAR, 2012 Phase II clinical study of VM202-DPN (Diabetic Peripheral Neuropathy medicine) approved by KFDA.
- MAR, 2012 NDA approval of PG201 (osteoarthritis medicine), which is the 7th botanical medicine to be approved in Korea
- MAR, 2012 Phase III clinical study of VM501 (Chemotherapy-Induced Thrombocytopenia medicine) approved by CFDA
- NOV, 2011 Phase II clinical study of VM202-DPN (Diabetic Peripheral Neuropathy medicine) approved by US FDA
- OCT, 2010 Phase II clinical study of VM202-PAD (Peripheral Artery Disease medicine) approved by KFDA
- NOV, 2009 Domestic launch of Allex, a nutraceutical designed to improve immune hypersensitivity and AtoLatte, a topical solution for atopic dermatitis
- JUN, 2008 China State Food and Drug Administration (SFDA) approval of Phase I clinical trial for peripheral artery disease (VM202-PAD)
- DEC, 2004 Start joint research efforts for biomedical products with Yuhan Corporation in Korea
- NOV, 1996 Incorporation of ViroMedica Pacific, Inc. (now ViroMed Co., Ltd.)
